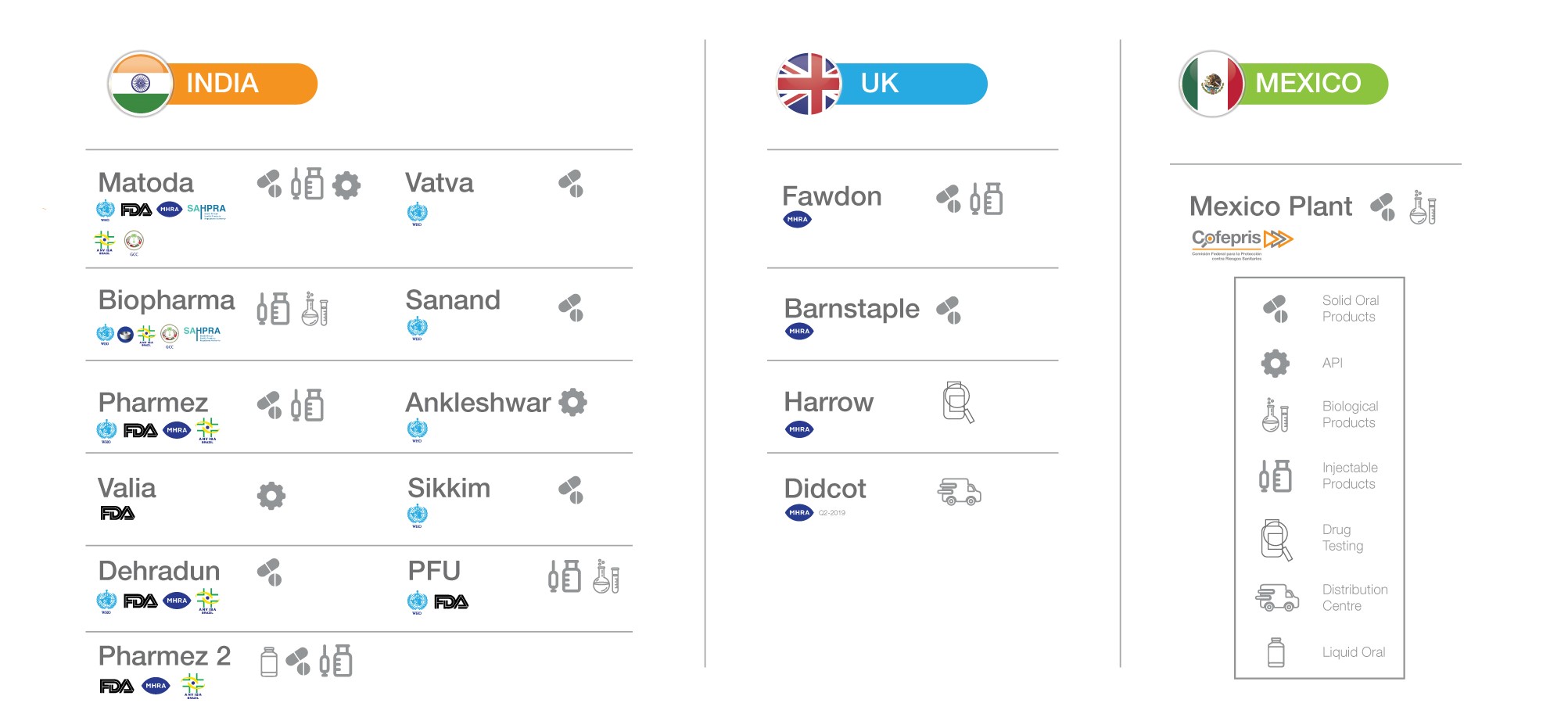
Manufacturing
Intas operates fourteen formulation manufacturing facilities, of which nine are located in India, and the rest in the U.K. and Mexico. It also operates two API and intermediate manufacturing facilities, each of which complies with the regulatory requirements in the jurisdictions in which it operates. Between them, these facilities have received approvals from various prominent international regulatory bodies, including from U.S. Food and Drug Administration (FDA).
Each of Intas’ manufacturing facilities is designed, equipped and operated to deliver high-quality products within defined cost and delivery schedules. These manufacturing facilities have the flexibility to operate in various dosage forms and a wide range of batch sizes. Intas’ world class oncology formulation facility operates under global regulatory and safety standards. The Ahmedabad SEZ caters exclusively to the US, Europe and other regulated markets. A highly advanced upcoming facility in Ahmedabad, Gujarat is set to double Intas’ manufacturing capabilities.
Manufacturing Facilities